- Description
- Discussions (0)
Description
1. Name of the medicinal product
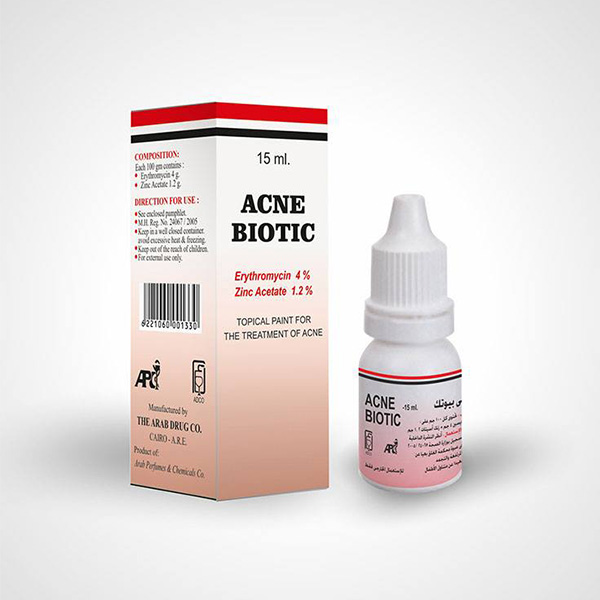
ACNE BIOTIC paint
2. Qualitative and quantitative composition
Erythromycin-zinc complex containing the equivalent of 40 mg/ml erythromycin and 12 mg/ml zinc acetate.
For the full list of excipients, see section 6.1.
3. Pharmaceutical form
Topical solution.
4. Clinical particulars
4.1 Therapeutic indications
Acne biotic is indicated in children, adults and the elderly. It is used as topical treatment of acne vulgaris.
4.2 Posology and method of administration
For cutaneous use. Apply twice daily over the whole of the affected area for a period of 10 to 12 weeks.
4.3 Contraindications
Hypersensitivity to the active substance(s), to other macrolide antibiotics, or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Cross resistance may occur with other antibiotics of the macrolide group and also with lincomycin and clindamycin. Contact with the eyes or the mucous membranes of the nose and mouth should be avoided.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Fertility, pregnancy and lactation
Acne biotic can be used during pregnancy and during breast-feeding (but it should not be used on the chest).
4.7 Effects on ability to drive and use machines
None.
4.8 Undesirable effects
Tabulated list of adverse reactions
| System Organ Class | Rare
>1/10,000, <1/1000 |
Very rare
<1/10,000, Not known (cannot be estimated from the available data) |
| Immune system disorders | Hypersensitivity | |
| Skin and subcutaneous tissue disorders | Pruritus
Erythema Skin irritation Skin burning sensation Dry skin Skin exfoliation |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
4.9 Overdose
It is not expected that overdosage would occur in normal use. Patients showing idiosyncratic hypersensitivity should wash the treated area with copious water and simple soap.
5. Pharmacological properties
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Anti-acne
Erythromycin is known to be efficacious, at 4%, in the topical treatment of acne vulgaris. Zinc, topically, is established as an aid to wound healing.
5.2 Pharmacokinetic properties
Absorption
The complex does not survive in the skin, and erythromycin and zinc penetrate independently. The erythromycin penetrates, and is partially systemically absorbed (0 – 10% in vitro, 40 – 50% in animal studies); that portion absorbed is excreted in 24 – 72 hours. The zinc is not absorbed systemically.
5.3 Preclinical safety data
Non-clinical data from repeated dose toxicity and reproduction and developmental toxicity studies reveal no additional hazard other than those described elsewhere.
6. Pharmaceutical particulars
6.1 List of excipients
Propylene glycol, Ethyl alcohol, Ethyl acetate
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years
6.4 Special precautions for storage
Store at temp. not exceeding 30˚C
Keep out of the reach of children.
6.5 Nature and contents of container
Self-dropper HDPE plastic bottle of 15 ml solution covered by HDPE plastic dropper and covered by HDPE plastic cover in acarton box with an insert.
6.6 Special precautions for disposal and other handling
No special requirements.
7. Marketing authorization holder
Arab Perfume, Chemicals &Pharmaceutical company.
8. manufactured by
Arab drug company










Discussions
There are no discussions yet.
Comments are closed.